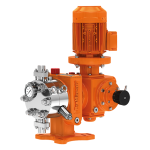
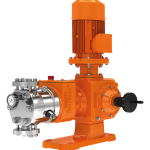
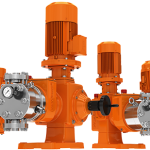
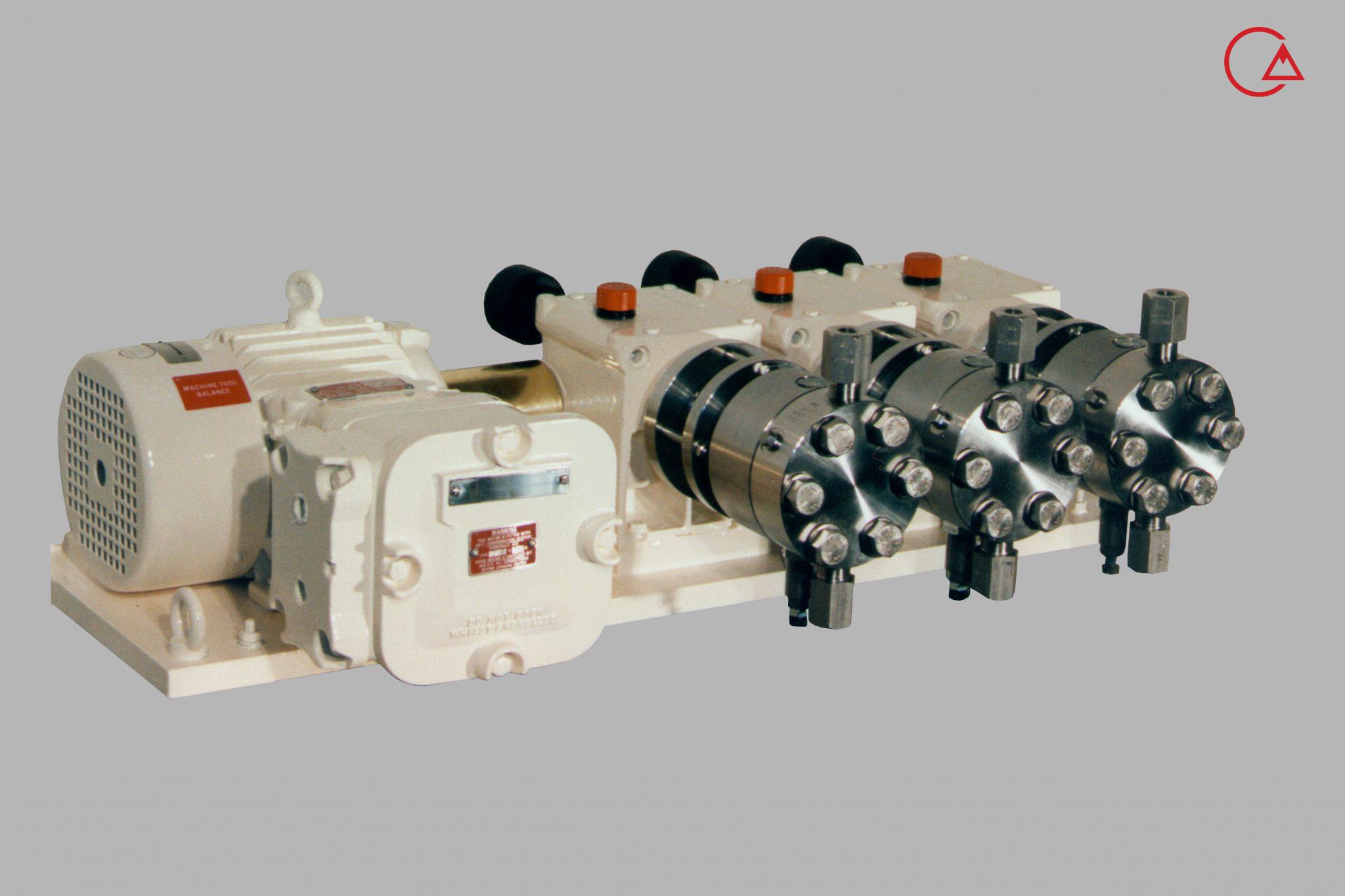
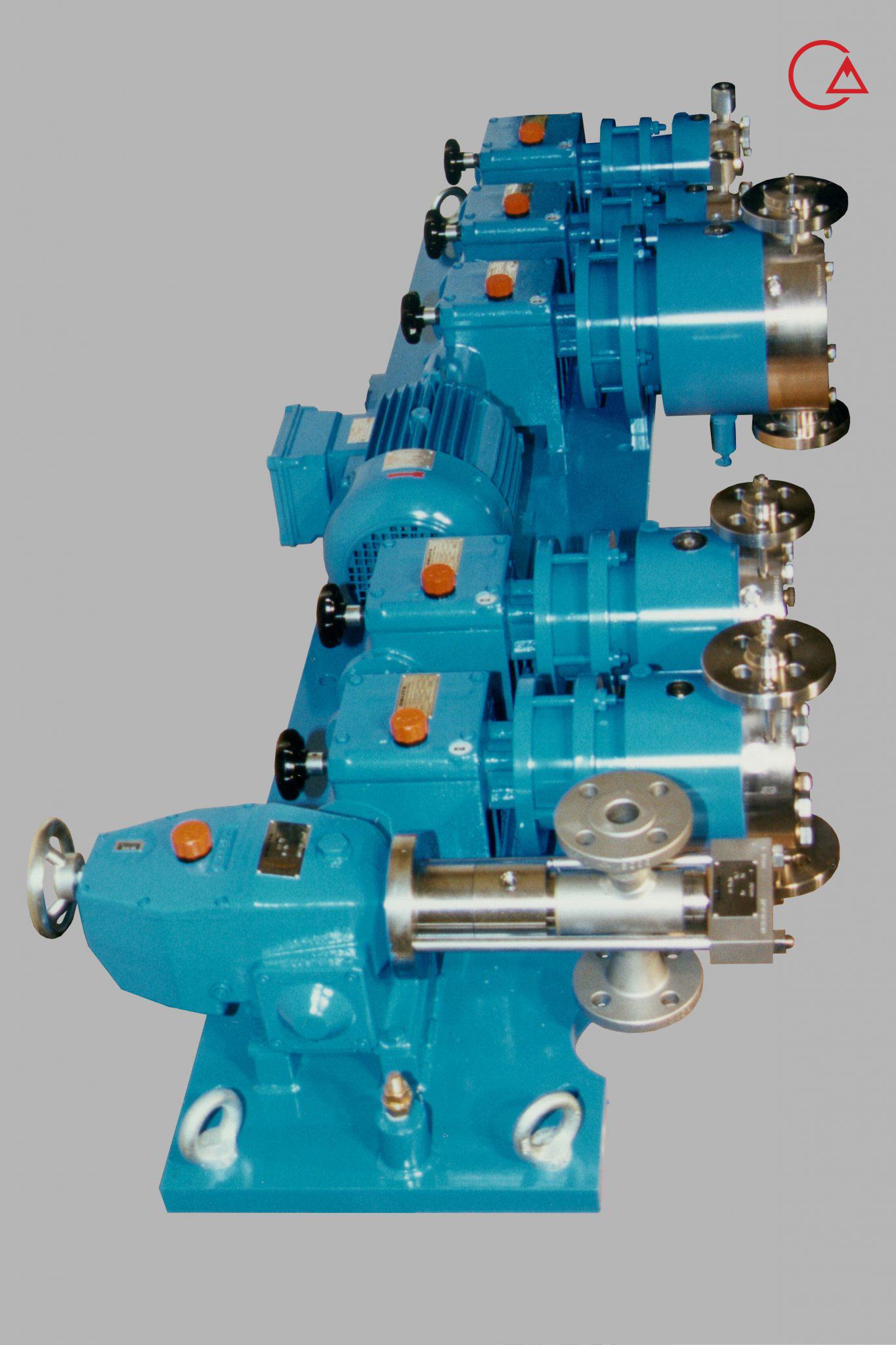
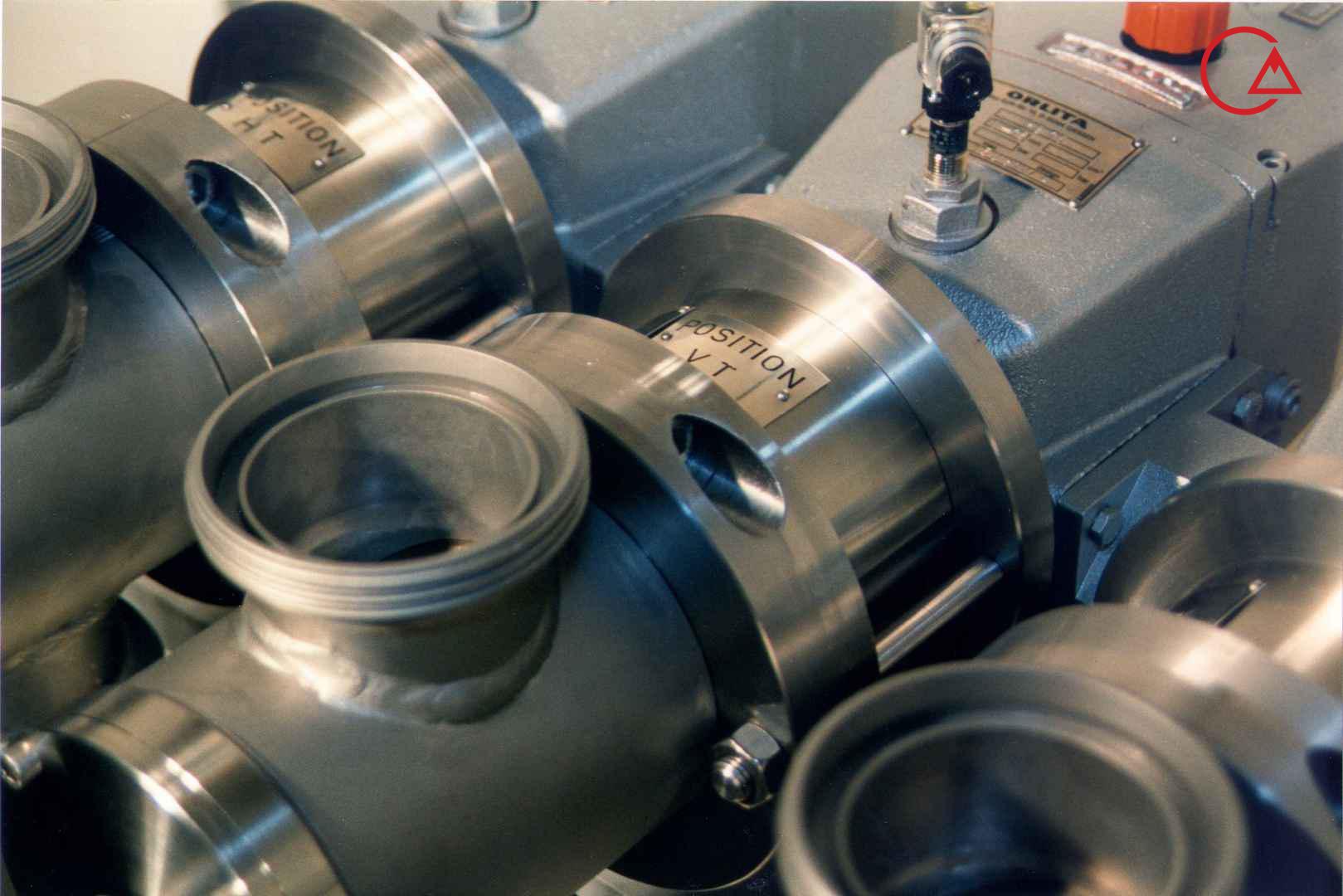
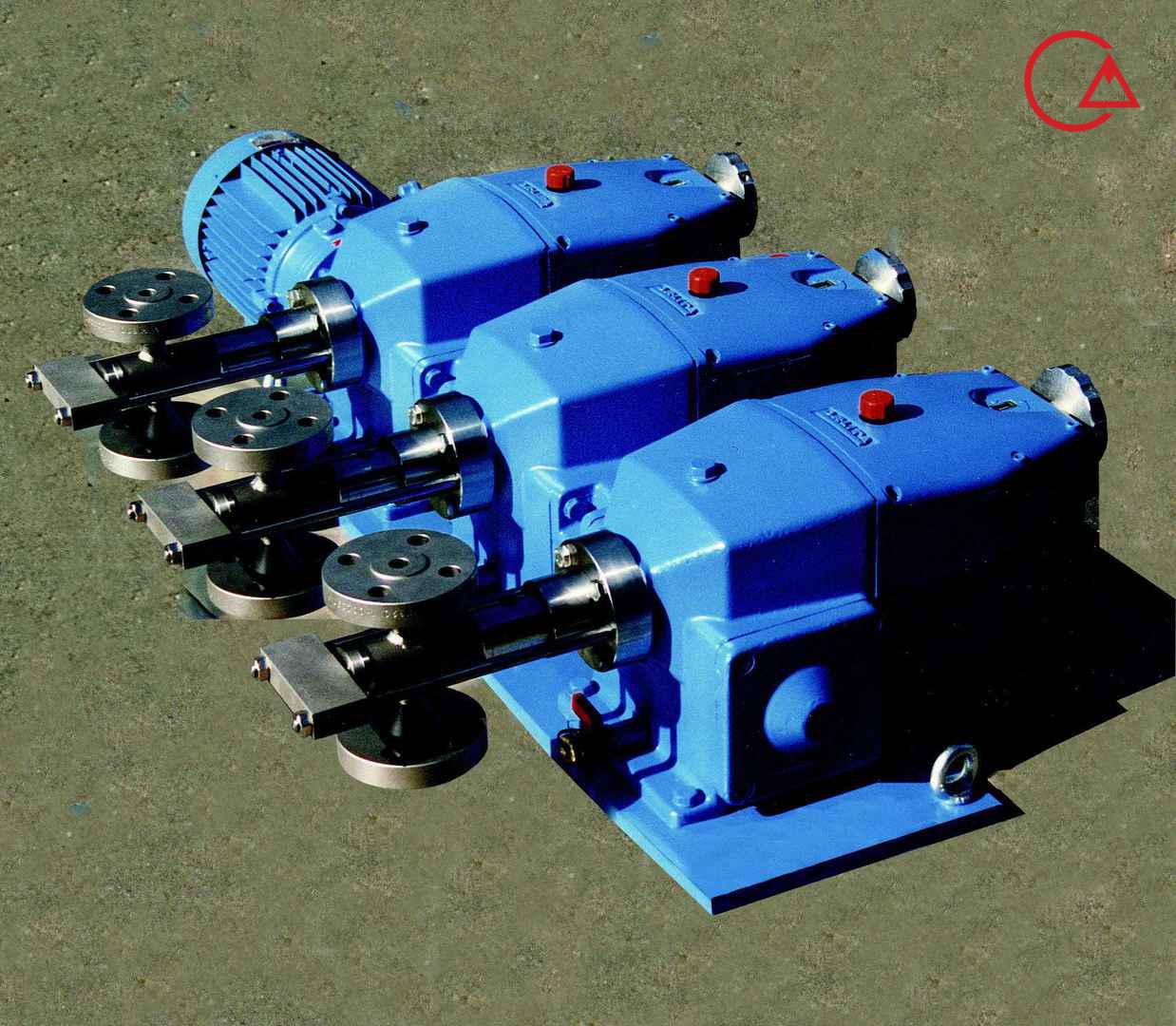
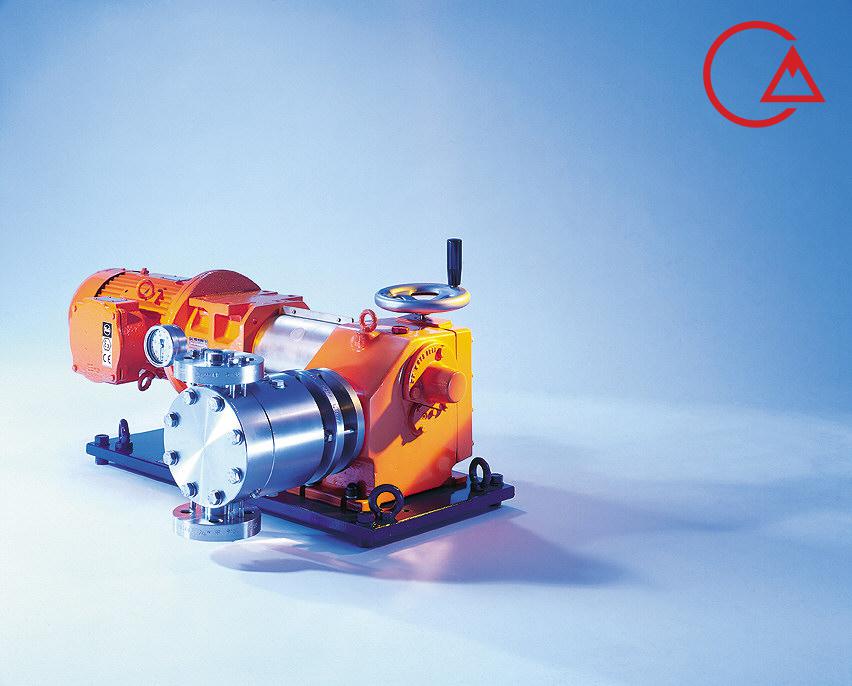
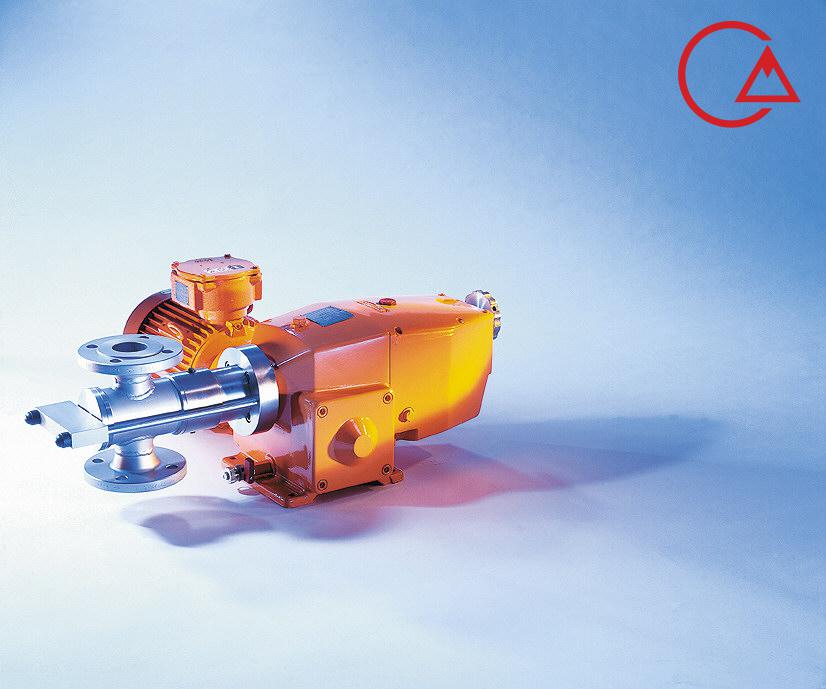
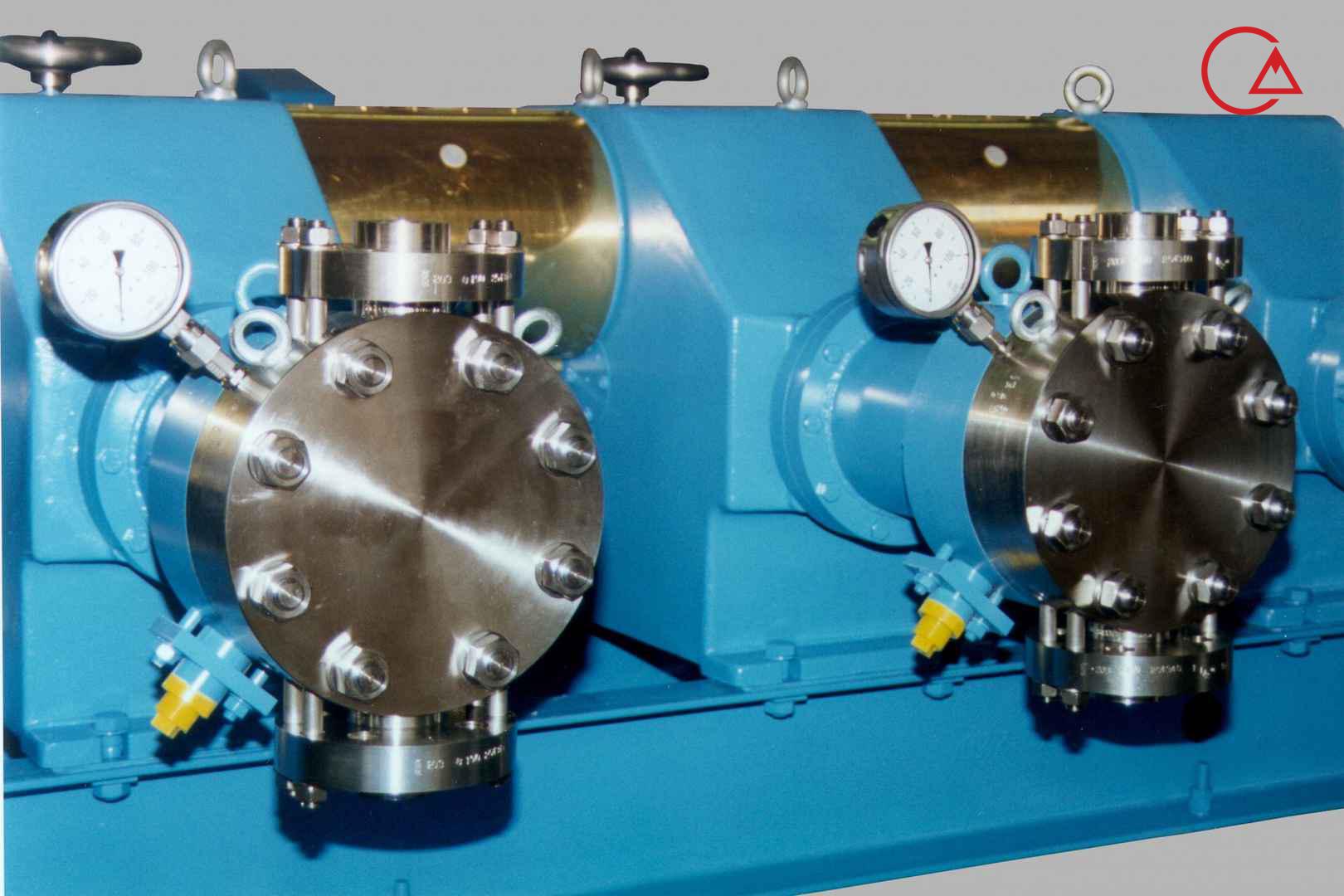
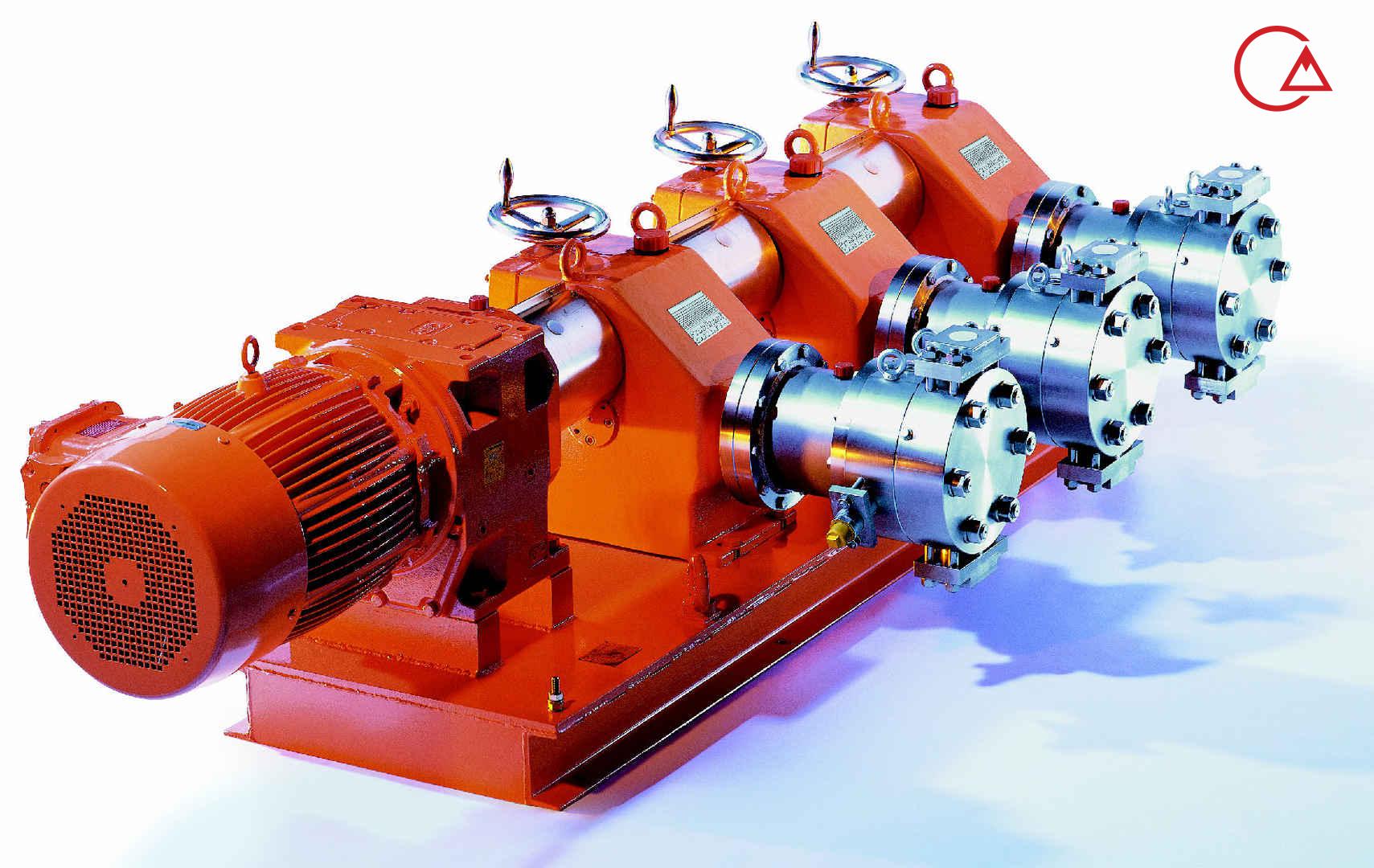
کمپانی اورلیتا در سال 1995 توسط پرومیننت خریده شد و به زیرمجموعه ای از این کمپانی تبدیل شد تا پرومیننت بتواند دوزینگ پمپ با استاندارد نفت و گاز امریکا API675 تولید کند.

اخیرا کمپانی پرومیننت اولین سری پمپ مناسب با این استاندارد را در مجموعه خودش به تولید رساند و نام آن را Evolution قرار داد.