Acid descaler or sediment germicidal mean?
Acid descaler (sediment germicidal) for all forms of life is essential, and in all the cells found. Nucleic acids in two forms normal to The دیاکسی ریبونوکلئیک acid (DNA) and acid ریبونوکلئیک (RNA) there are.
Nucleic acids of polymers Bio are made naturally, a plethora frequent مونومرها (polymers manufacturer) chemicals that are then نوکلئوتیدهایی to create that nucleic acids forming.
To understand the structure of the nucleic acid, etc. understand the structure نوکلئوتیدهایی that the nucleic acid make up is very important.
The sale of acid descaler or sediment in an
Acid descaler (sediment germicidal) molecules biodiversity are of great that play a fundamental role in all cells and viruses are. The main function of nucleic acids involves the storage and expression of information, genomic is. De Oxy ریبونوکلئیک acid, or DNA, of the information which cells build proteins require encryption does. A type of nucleic acid, etc. to the name of the acid ریبونوکلئیک (RNA), etc. بایوساید in different shapes, molecular, there is the role of the cellular numerous, including protein synthesis, it plays.
A variety of acid descaler (sediment members)
Acid descaler (sediment germicidal) polymer molecule with a long chain? are monomers (single recurring) as the nucleotide known, and hence sometimes nucleic acids to Poly-nucleotides say.
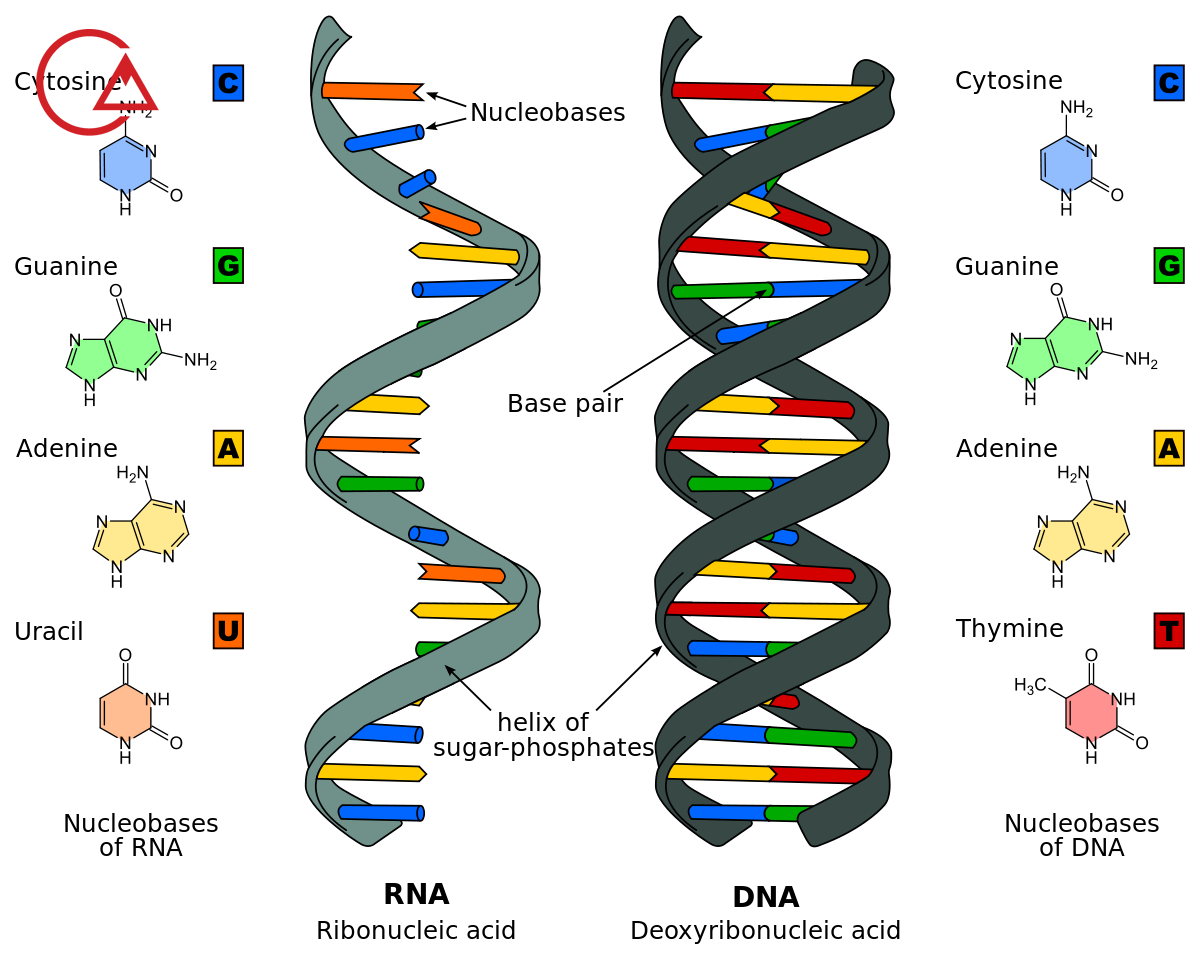
Deoxyribonucleic acid (DNA) and acid ریبونوکلئیک (RNA) are the two main types of nucleic acids are. DNA and RNA, Poly aluminium chloride, responsible for the inheritance and the transfer of the special features from one generation to another. An outstanding two types of nucleic acid, is known to us.
The structure of the nucleic acid
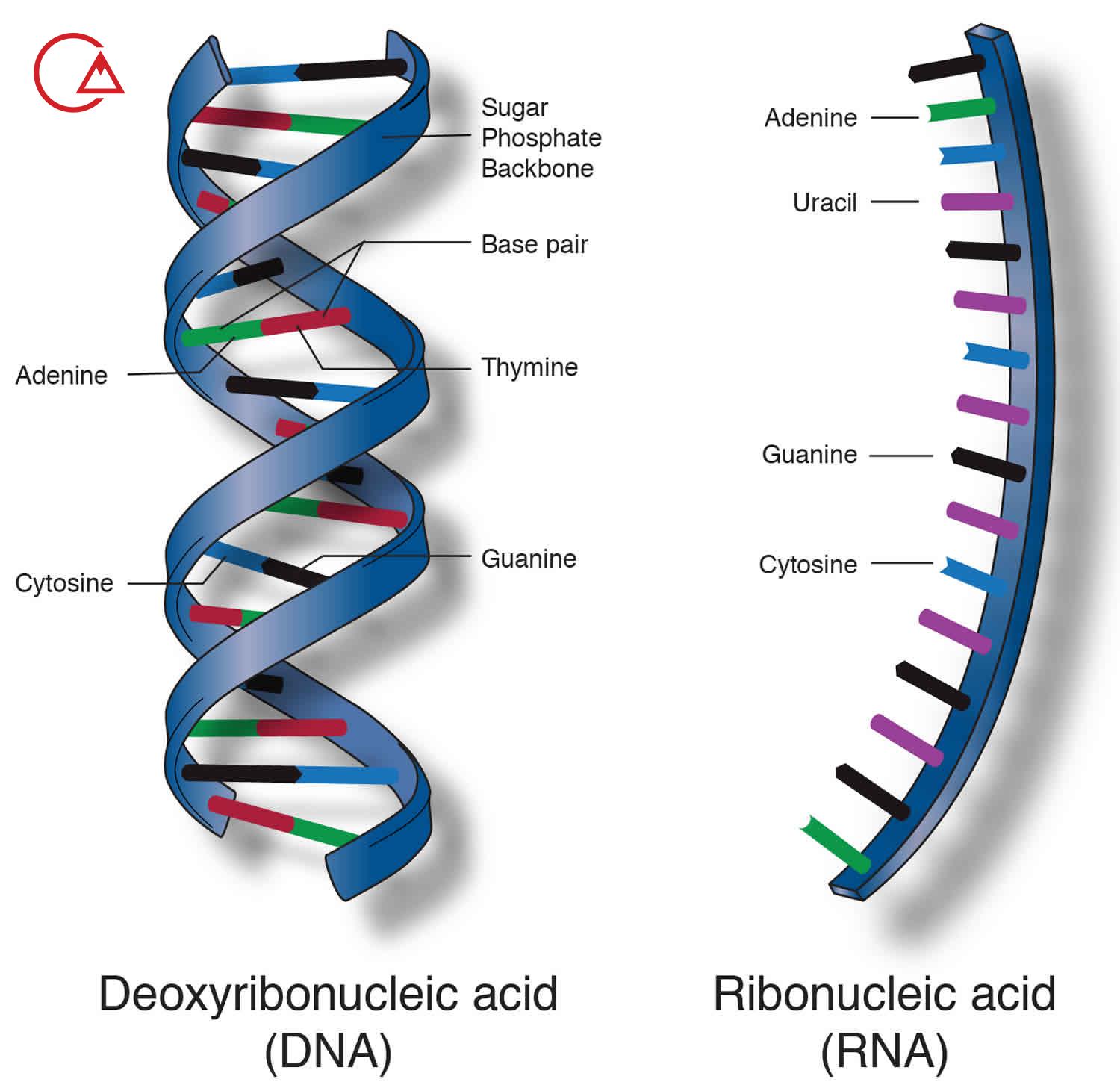
An acid descaler (sediment commission ( composed of three parts, which by the links to be connected have been. This episode includes a group of phosphate, a sugar, 5 کربنه and a basic nitrogen.
Group phosphate
Group phosphate of one atom of phosphorus is formed by four oxygen atoms with a negative charge attached to it.
Sugar 5 کربنه
Sugar 5 کربنه (known as پنتوز) contains ribose and deoxyribonucleic ribose, which is acid descaler (sediment germicidal) there. Ribose and deoxyribonucleic ribose both have five carbon atoms and one oxygen atom are. To carbon atoms, atoms of hydrogen and hydroxyl are connected.
In the sugar ribose, etc. contain hydroxyl groups attached to the atom, the second and Third carbon there. In sugar deoxyribonucleic ribose, a group hydroxyl attached to the carbon atom, there is a third, but only one hydrogen atom to a carbon atom, the second is connected.
Base nitrogen
A molecule of nitrogen as a base in acid descaler (sediment members) acts, because it can be other molecules, the electrons give, and through this process, the molecules will create new. Can molecules of carbon, hydrogen and oxygen connected to the structure of the rings make.
The structures of the ring, for Ring, single (پیریمیدین) and ring dual (purine) are. پیریمیدین include biological functions سیتوزین and L-RNA are. Purine include آدنین and guanine are. Purines are the larger of the پیریمیدین are, and the difference of their size, to determine their pairing in DNA strands can help.
Links nucleic acid
The links that molecules P, sugar and nitrogen, together, hold, etc. the links گلیکوزیدی and links Astrea called.
Links گلیکوزیدی between the first carbon atom in a sugar 5 کربنه and the ninth nitrogen atom in a base, the nitrogen can be created.
Links Astrea between the fifth carbon atom in glucose 5 کربنه and groups, phosphate can be created.
These links not only a nucleotides together and they hold, but the chain of nucleotides, also together, hold that the نوکلئوتیدهایی make that deoxyribonucleic acid (DNA) and acid ریبونوکلئیک (RNA) make up.
To create this زنجیرهها, Department of phosphate that to the fifth carbon atom in a sugar 5 carbon is connected to the carbon atom third sugar 5 carbon of the next connected. This is repeated up the chain created by a backbone of sugar-phosphate is connected.
If sugar is available in this chain, a glucose, ribose, is. a string of RNA, it is created.
To create DNA. the RNA strand is made of nucleotides connected that structurally similar, but anti-parallel with links to the name of the hydrogen bonding is. This links the hydrogen پیریمیدینها and پورینهای available in foundations of نیتروژنی to the link, they are. In a process called pairing bases, supplements, etc. guanine to سیتوزین And آدنین to تیمین link knocks. This increases the energy efficiency of the pairs, the base is always in this pattern are found.
The performance of nucleic acid
- Nucleic acids are responsible for the transfer of inherent characteristics from parents to children are.
- They are responsible for protein synthesis in the body we are
- Fingerprinting DNA is a method by forensic experts to determine the parents used. Also, to identify the culprits used. Also plays an important role in studies related to biological evolution, and genetics have been.
Any type of nucleic acid, the performance of different in the cells of all living organisms do.
Deoxyribonucleic ریبونوکلئیک acid (DNA)
In terms of chemical, DNA from a sugar پنتوز., the phosphoric acid, and some hawks, the annular containing nitrogen is formed. Part of sugar contained in molecules of DNA, β-D-2-deoxyribonucleic ribose is. Hawks circular that the nitrogen in them there are آدنین (A), guanine (G), The سیتوزین (C) and timin(T). The bases and makeup them in the molecules of DNA plays an important role in the storage of information from one generation to another. DNA structure double helix is a sequence in which the fields complement each other.
DNA is responsible for storing and coding genetic information in the body. The structure of DNA to allow children the genetic information of their parents to inherit the Earth.
Since the nucleotides آدنین., the biological functions guanine and سیتوزین in DNA, just in a specific sequence pairs are (آدنین with تیمین and guanine with سیتوزین), every time that cellular DNA to reproduce does, you can sequence the nucleotides must be in it the whole…. determine. Copy. As such, copies a detailed analysis of the DNA can be made and handed down from generation to generation, is passed on.
Inside the DNA. guidelines for all the proteins that an organism makes to be stored.
Acid ریبونوکلئیک (RNA)
RNA molecule is also of phosphoric acid, etc., sugar, پنتوز and some hawks, the annular containing nitrogen is formed . RNA has a β-D-ribose in it as part sugar. Hawks heterocyclic found in RNA are آدنین (A), guanine (G), The سیتوزین (C) and uracil – (U). In RNA, the base of the fourth with DNA is different. RNA is generally a Rasht formed, and that sometimes rear up to be. Which leads to the structure of the double helix can be. Three type of RNA molecule that exists, each of which yield certain:
This functions by RNA with a different name, done. These names include:
- RNA messaging (m-RNA)
- RNA nested PCR (r-RNA)
- Transfer RNA (t-RNA)
RNA plays an important role in protein synthesis plays and expression information is stored in DNA to the construction of these proteins regulates. Also, how to transfer genetic information in some viruses.
- Different tasks RNA include:
- Create new cells in the body
- Convert DNA to protein
- As a messenger between DNA and ریبوزوم acts
- To ریبوزوم helps amino acids, suitable for the creation of proteins in a new body, their choice.
ATP
However, all the acid, descaler (sediment members) in the processing of information is stored in the cells, the role of Don’t. Nucleic acid adenosine triphosphate (ATP), which is an open nitrogenous آدنین, a sugar, ribose 5-carbon and three phosphate groups and in the production of energy for cellular processes play a role.
The links between the three groups, phosphate, nitrate, links, energetic and are energy the cell can provide. All living cells of ATP for energy use up to allow them to perform their duties will lose.
For the supply of energy, the last group of phosphate in the chain is removed, that the free energy does. This process of ATP to adenosine diphosphate (ADP) changes. Remove the two groups of phosphate from ATP, the energy required to create adenosine monophosphate (AMP) Production.
ATP can be again through a process of recycling in the mitochondria of the village that the phosphate groups charged again and again to the chain adds.
ATP in the transfer of proteins and lipids into and out of the cells, the role of which is to arrange to name اندوسیتوز And اگزوسیتوز are known.
The ATP also in maintaining the overall structure of a cell is important because to build properties, structure, cell-cell help.
In terms of special functions on the body, but ATP in muscle contraction is important. This includes the contractions created by the heart during the pounding and also the movements that is larger muscle groups can be done.
The benefits of acid descaler (sediment members)
- Has the power of removing sediment is very high
- Tail damage to the environment and has a lower risk
- No need for thermal energy and heat
- Has high capacity acid descaler the direction of the transport layer of the oxide
- Has the ability of removing sediment, while being clear on the device
- Non-toxic, being
- Has the ability, lack of injury, a metal surface installations and the lack of corrosion, they
FAQ :
Acid descaler (sediment germicidal) what?
Nucleic acids natural chemical compounds are as molecules, the primary carrier of information in the cells of the act. They play an important role in guiding the synthesis of protein are. Two main categories of nucleic acids include deoxyribonucleic ریبونوکلئیک acid (DNA) and acid ریبونوکلئیک (RNA).
Does the DNA of an acid descaler is?
Nucleic acids, etc. deoxyribonucleic acid (DNA) and acid ریبونوکلئیک (RNA), the carrier of genetic information that are in the cells is read to RNA and پروتئینهایی to build that organisms by their work.
Acid descaler 4 What?
نوکلئوزیدهای modified chemical derived from derivatives ریبو or deoxyribonucleic ریبونوکلئوزید conventional adenosine, etc. سیتوزین, etc. گوانوزین, and یوریدین or تیمیدین in all types of nucleic acids, etc., DNA and RNA are found.
Acid descaler (sediment germicidal) with example, what is it?
Two examples of nucleic acids include deoxyribonucleic acid (better as DNA known) and acid ریبونوکلئیک (better as RNA known to be). The molecules of Rasht, long نوکلئوتیدهایی formed, and which by the links covalent beside. Nucleic acids can be found in the nucleus and cytoplasm of our cells found.
Display video
List price acid descaler
MSDS acid descaler
Acid descaler (sediment germicidal) is an essential part of all living organisms and is the building block for DNA and RNA. In all the cells and also in some viruses found. Nucleic acids set of functions are very diverse, they have, such as creating cells, store, and the processing of genetic data, building proteins and producing energy cells Weg.
Although their performance may vary., the structures of DNA and RNA are very similar, and only a few fundamental difference in the molecular structure of them makes a distinction they can be.
