Advantages of salt electrolysis
- It contains significantly less salt than tap water.
- Reduction of transport costs for raw materials to oil refineries.
- Reducing the costs of supplying consumer chemicals.
- Safety and harmless properties of chlorine gas
- Stability of the disinfectant
- Low by-product content
- Easy access to raw materials
- Reduce contact time and use disinfectant throughout the network.
- This does not increase the pH value of the water.

Application of salt electrolysis
- Drinking water treatment in cities
- Water treatment in sports centers and residential areas
- Water treatment for swimming pools and fountains
- Water treatment for industrial processes such as cooling towers.
- Water treatment for food and beverage production.
- Wastewater treatment and recycling
Consumables for salt electrolysis plants
- Salt
- Water
- Current flow
The amount of salt consumed is calculated in direct proportion to the water volume and fed into the reactor of the device.
While an electric current flows through the reactor, sodium hypochlorite and finally hydrogen are removed from the apparatus and stored in tanks.
Sodium chloride + H₂O ———–> NaOCl + H₂
Phases of the electrolysis process
- The required amount of salt is calculated in real time and injected into the reactor.
- The flow of electric current causes the process of electrolysis.
- The juice is bottled as a finished product in metal cans.
- The water obtained using the Javier method is used for water purification.
He represents
Given the increasing demand for reliable and sustainable water disinfection across various sectors, traditional methods such as chlorination or the use of premixed sodium hypochlorite face numerous challenges, including safety risks, high transportation costs, and chemical instability. In contrast, brine electrolysis (also known as electrochlorination ) is gaining increasing importance as a safe, cost-effective, and modern technology in water treatment and disinfection systems.
The system uses salt (NaCl) and electricity to produce a sodium hypochlorite solution on site for disinfecting drinking water, process water, wastewater and swimming pool water.
What is an electrochlorination device?
An analytical chlorinator is a device that generates active chlorine through the electrolysis of a salt solution . Chlorine ions from the salt are converted into highly effective disinfectants using an electric current.
The end product of this process is typically sodium hypochlorite in low concentration (0.6% to 1%), which can be introduced into the system immediately without the need for long-term storage.
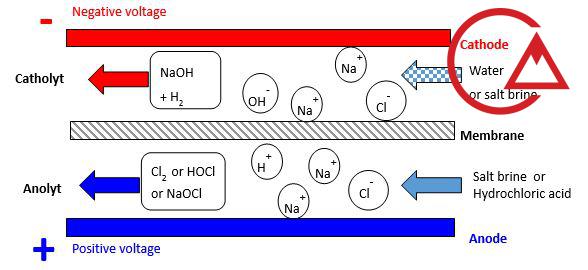
The operating principle of the salt electrolysis plant.
The operating principle of the electrochlorination device is based on an electrochemical reaction in an electrolysis cell:
Salt is dissolved in water to create a salt solution.
The solution passes through an electrolysis cell.
Apply direct current to the electrodes.
Release of active chlorine and formation of sodium hypochlorite.
The resulting solution is injected into the target water stream.
In general, the answer can be formulated as follows:
NaCl + H₂O + electrical energy → NaOCl + H₂
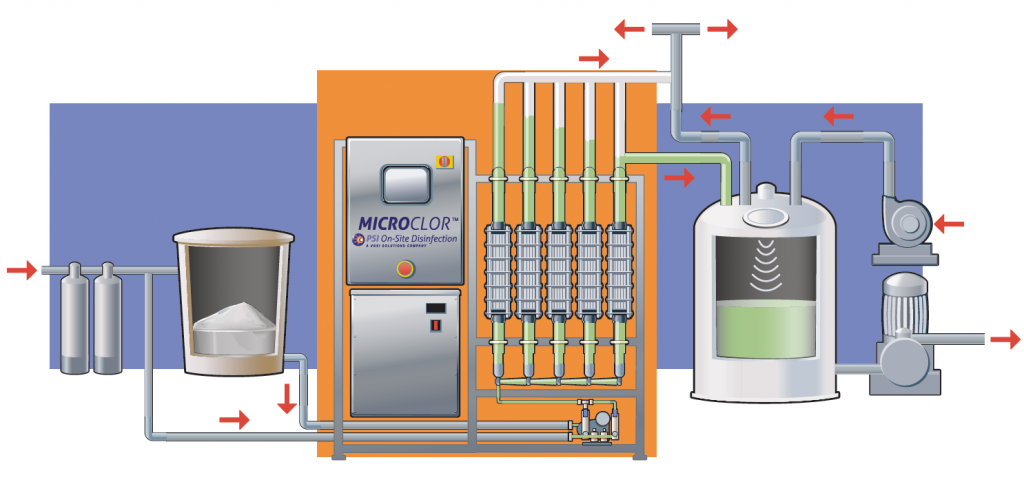
The main components of an electrolytic chlorination system
The following components are typically included in a salt electrolysis plant:
Brine tank
Electrolysis cells with titanium-coated electrodes.
Power supply and rectifier (AC to DC)
Distribution boxes and intelligent control systems
high-pressure pump or dosing system
Safety systems and control sensors
These components are manufactured as a single unit, which greatly simplifies installation and operation.
Types of electrochlorination equipment
Electrolytic chlorination plants can be divided into the following categories depending on their application and capacity:
1. Electrochlorine sterilizer for drinking water
Target audience:
Urban and rural water treatment plants
Pumping station
Water supply systems
2. Industrial electrochlorination plant
It is used for:
Oil, gas and petrochemical industry
power plant
Food and pharmaceutical industries
3. Electric chlorinators for swimming pools and leisure centers.
To:
pool
Water park
Sports center
Advantages of using salt electrolysis plants
Compared to conventional methods, the use of an electrochlorination device offers the following advantages:
 extremely high level of security
extremely high level of security
It is not necessary to use dangerous chlorine gas or concentrated chemicals .
 Autonomous chlorine generator
Autonomous chlorine generator
Eliminates transport and storage costs as well as the risk of leaks.
 Low operating costs
Low operating costs
Salt is a cheap and readily available raw material.
 Consistent sterilization quality
Consistent sterilization quality
New production of highly effective hypochlorite
 Environment
Environment
Reduction of chemical waste and harmful gas emissions.
Comparison of electrochlorination plants and traditional chlorination methods.
| Street | Protection | It’s worth it | Sustainable development | Save |
|---|---|---|---|---|
| chlorine | Very low | higher | half | High risks |
| Hypochlorite | half | half | fewer | Storage required. |
| Analytical Chlorinator | Very large | fewer | higher | base |
Application of electrolysis plants for the production of salts.
Analytical chlorination equipment is widely used in various projects:
Drinking water treatment in urban and rural areas.
Wastewater must be disinfected before disposal.
Industrial cooling systems
Water circulates in the cooling tower .
Food, dairy and beverage industry
Swimming pool and whirlpool
Important points to consider when selecting a chlorination analyzer.
The following points should be taken into account when selecting suitable salt electrolysis systems:
Chlorine production capacity (g/hour)
Quality and type of electrodes
Control and automation systems
Energy consumption
Customer service and spare parts supply.
Making the right choice extends the lifespan of your equipment and reduces maintenance costs.
Maintenance and operation
The maintenance of analytical chlorination plants is relatively simple and includes the following:
Regular checks of the electrodes
If deposits are present, the cells should be washed.
Salt concentration control
Check the functionality of the distribution box.
If you follow these recommendations, your equipment can last for more than 10 years.
In conclusion
Salt electrolysis plants ( electrochlorination plants) are among the newest and safest methods of chlorine production for water disinfection and are suitable for disinfection on various scales. This system is based on on-site production technology, which reduces costs and safety risks while ensuring consistent and controllable disinfection results.
Today, the use of electrochlorination plants is no longer just a technological option, but a sensible economic and ecological solution for industrial companies and plants.