پمپ های صفحه ای تکان دهنده
| | محور محرک | | دیافراگم ها |
| | رولبرینگ مخروطی | | مجموعه دریچه مکش |
| | بادامک با زاویه ثابت / صفحه تکان | | مجموعه دریچه تخلیه |
| | سلول های هیدرولیک (پتنت شده) | | شیر تنظیم فشار |
قابل اعتماد، پمپاژ کارآمد
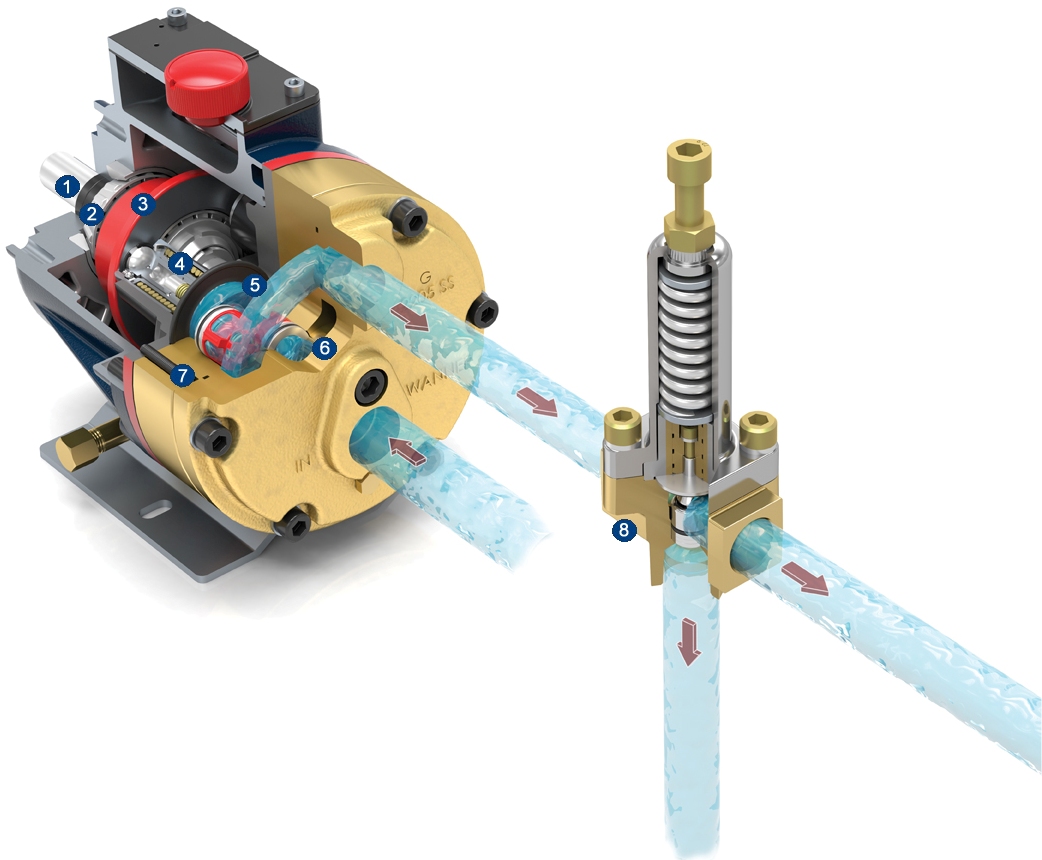
محور محرک (1) توسط یک غلتک مخروطی بزرگ در عقب شفت و یک یاتاقان کوچکتر (2) در جلوی شفت در جای خود نگه داشته می شود. بین یک جفت دیگر یاتاقانهای بزرگ یک بادامک یا صفحه متلاطم با زاویه ثابت (3) قرار دارد.
با چرخش محور محرک، صفحه تاب خورده به سمت جلو و عقب نوسان می کند و حرکت محوری را به حرکت خطی تبدیل می کند. مجموعه کامل شفت/صفحه تکان دهنده در حمام روغن روان کننده غوطه ور می شود.
سلول هیدرولیک (4) به صورت متوالی توسط صفحه تاب خورده حرکت می کند و در حرکت رو به عقب آن با روغن پر می شود. یک شیر چک توپ در پایین سلول هیدرولیک تضمین می کند که در حرکت رو به جلو پر از روغن باقی می ماند.
روغن نگهداشته شده در Hydra-Cell به قسمت پشتی دیافراگمها فشار وارد میکند و باعث میشود که دیافراگمها به سمت جلو و عقب در حین حرکت صفحه تاب خورده خم شوند. این عمل پمپاژ را فراهم می کند.
Hydra-Cell برای ارائه عمر طولانی بدون مشکل دیافراگم، دیافراگم ها (5) را در محدوده فشار کامل پمپ به صورت هیدرولیکی متعادل می کند. دیافراگم بدون توجه به فشاری که سیال در آن تحویل داده میشود، تنها با اختلاف فشار 3 psi/0.2 بار مواجه است – تا 172 بار در مدلهای استاندارد Hydra-Cell و پمپهای اندازهگیری Hydra-Cell.
پمپ های صفحه متحرک Hydra-Cell می توانند تا پنج دیافراگم داشته باشند، هر دیافراگم دارای محفظه پمپاژ مخصوص به خود است که شامل یک مجموعه شیر چک دیسک افقی خود تراز شده مکش و تخلیه است (6). با حرکت دیافراگم ها به عقب، مایع از طریق یک ورودی مشترک وارد پمپ می شود و از یکی از شیرهای چک مکش عبور می کند. در حرکت رو به جلو، دیافراگم سیال را از شیر چک تخلیه (7) و از طریق خروجی مشترک منیفولد خارج می کند. دیافراگم ها به طور مساوی از یکدیگر فاصله دارند و به ترتیب عمل می کنند تا جریان ثابت و کم پالس را فراهم کنند.
یک شیر تنظیم فشار Hydra-Cell (8) اغلب در سمت تخلیه پمپ برای تنظیم فشار فرآیند یا تجهیزات پایین دست و محافظت از سیستم در برابر فشار بیش از حد نصب می شود.
