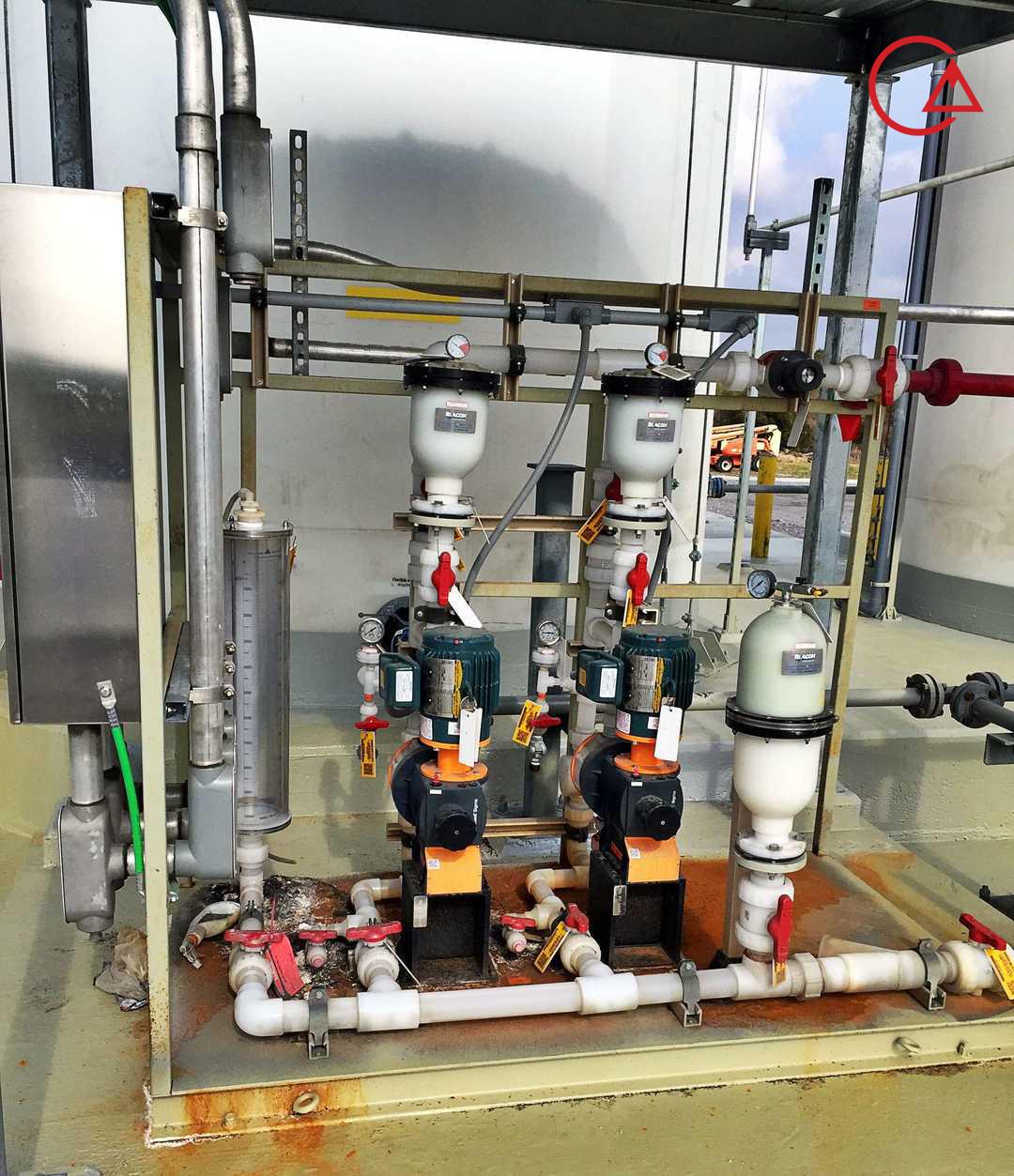
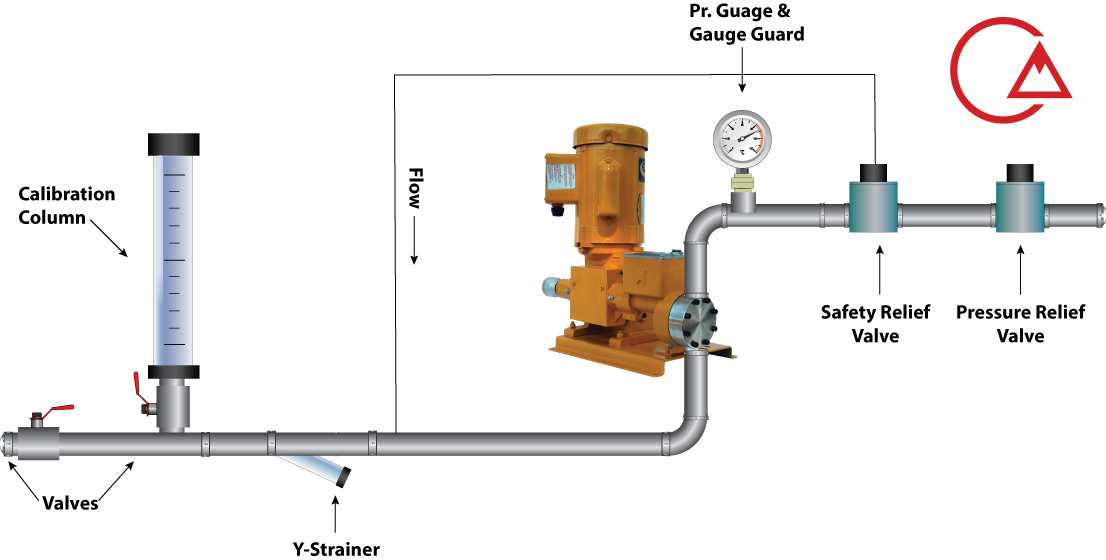
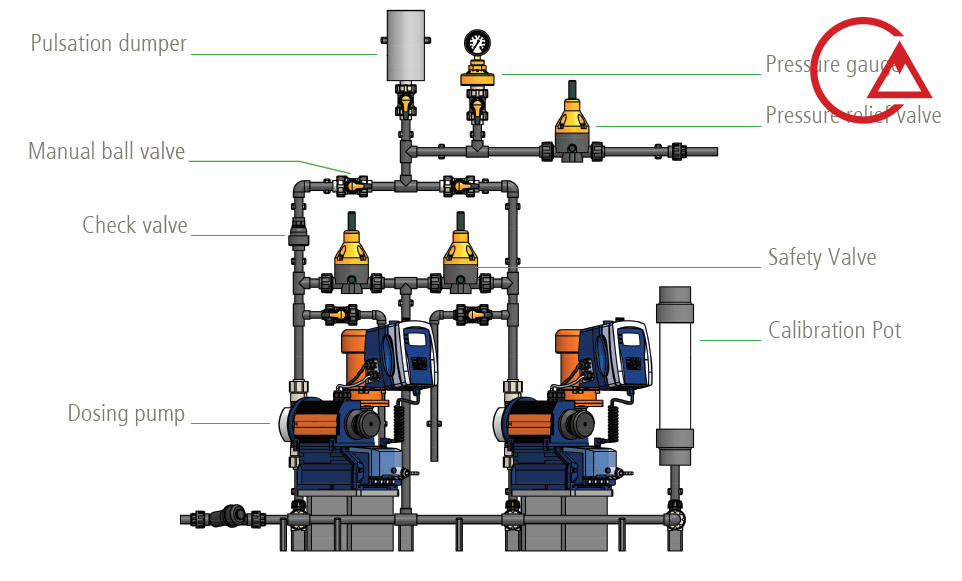
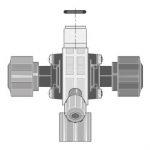
این شیر که با نام های مختلف خوانده می شود وظیفه حفظ ایمنی و محافظت از پایپینگ و دوزینگ پمپ را با جلوگیری از بالا رفتن بی رویه فشار ایفا میکند.
نام های دیگر این شیر:
- شیر اطمینان
- شیر اورفلو
- Pressure Safety Valve
- PSV
- Pressure Relief Valve
اصول شیر تخلیه فشار
شیر تخلیه یا شیر تخلیه فشار ( PRV ) نوعی شیر اطمینان است که برای کنترل یا محدود کردن فشار در یک سیستم استفاده می شود. در غیر این صورت ممکن است فشار ایجاد شود و باعث اختلال در فرآیند، خرابی ابزار یا تجهیزات یا آتش سوزی شود.
فشار با اجازه دادن به سیال تحت فشار از یک گذرگاه کمکی به خارج از سیستم کاهش می یابد. شیر کمکی طوری طراحی یا تنظیم شده است که با فشار تنظیم شده از پیش تعیین شده باز شود تا از مخازن تحت فشار و سایر تجهیزات در برابر فشارهایی که بیش از حد طراحی آنها باشد محافظت کند.
هنگامی که از فشار تنظیم شده فراتر رفت، شیر تسکین به «مسیری با کمترین مقاومت» تبدیل میشود، زیرا شیر به اجبار باز میشود و بخشی از سیال از طریق مسیر کمکی منحرف میشود.
سیال منحرف شده (مایع، گاز یا مخلوط مایع-گاز) معمولاً از طریق یک سیستم لولهکشی به نام فلر هدر یا هدر کمکی به یک فلر گاز مرکزی و مرتفع هدایت میشود که در آنجا معمولاً سوزانده میشود و گازهای حاصل از احتراق به اتمسفر رها میشوند. .
با انحراف سیال، فشار داخل ظرف افزایش نخواهد یافت. هنگامی که به فشار نشست مجدد شیر رسید، دریچه بسته می شود. دمش معمولاً به عنوان درصدی از فشار تنظیم شده بیان می شود و به میزان کاهش فشار قبل از قرار گرفتن مجدد شیر اشاره دارد.
دمش می تواند بین 2-20٪ متفاوت باشد و برخی از شیرها دارای دمش قابل تنظیم هستند.
شیر فشار
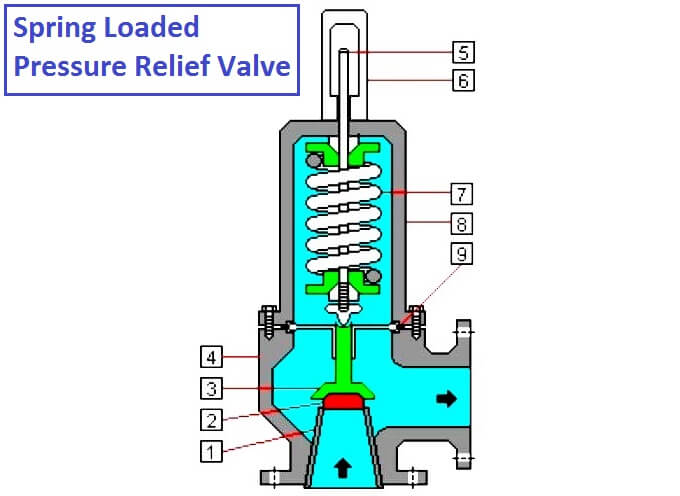
قطعات شیر تسکین دهنده:
- نازل ورودی،
- صندلی سوپاپ،
- نگهدارنده صندلی،
- بدنه سوپاپ،
- تنظیم پیچ تنظیم فشار،
- کلاه لبه دار،
- بهار،
- کاپوت،
- مهر
در سیستم های گاز فشار قوی توصیه می شود که خروجی شیر تخلیه در هوای آزاد باشد. در سیستمهایی که خروجی به لولهکشی متصل است، باز شدن یک شیر کمکی باعث ایجاد فشار در سیستم لولهکشی پایین دست شیر تخلیه میشود . این اغلب به این معنی است که پس از رسیدن به فشار تنظیم شده، شیر تسکین دوباره نمینشیند.
برای این سیستم ها معمولاً از شیرهای تسکین “دیفرانسیل” استفاده می شود. این بدان معنی است که فشار فقط در ناحیه ای کار می کند که بسیار کوچکتر از ناحیه دهانه شیر است. اگر دریچه باز باشد فشار باید قبل از بسته شدن شیر به شدت کاهش یابد و همچنین فشار خروجی شیر می تواند به راحتی شیر را باز نگه دارد.
نکته دیگر این است که اگر سایر شیرهای کمکی به سیستم لوله خروجی متصل شوند، ممکن است با افزایش فشار در سیستم لوله اگزوز باز شوند. این ممکن است باعث عملکرد ناخواسته شود.
در برخی موارد، شیر بایپس بهعنوان یک شیر کمکی عمل میکند و از آن برای بازگرداندن تمام یا بخشی از سیال تخلیه شده توسط پمپ یا کمپرسور گاز به مخزن ذخیره یا ورودی پمپ یا کمپرسور گاز استفاده میشود. این کار برای محافظت از پمپ یا کمپرسور گاز و هرگونه تجهیزات مرتبط در برابر فشار بیش از حد انجام می شود.
شیر بای پس و مسیر بای پس می تواند داخلی (بخش جدایی ناپذیر پمپ یا کمپرسور) یا خارجی (به عنوان جزئی در مسیر سیال نصب شود). بسیاری از موتورهای آتش نشانی دارای چنین شیرهای کمکی هستند تا از فشار بیش از حد شلنگ های آتش نشانی جلوگیری کنند.
در موارد دیگر، تجهیزات باید در برابر قرار گرفتن در معرض خلاء داخلی (یعنی فشار کم) که کمتر از توان تحمل تجهیزات است محافظت شوند. در چنین مواردی، از شیرهای خلاء برای باز شدن در یک حد فشار پایین از پیش تعیین شده و ورود هوا یا گاز بی اثر به تجهیزات به منظور کنترل میزان خلاء استفاده می شود.
نکات مهم :
اصطلاح شیر تسکین با اصطلاحات شیر تخلیه فشار (PRV)، شیر اطمینان فشار (PSV) و شیر ایمنی مرتبط است:
شیر تخلیه فشار (PRV) یا شیر تخلیه فشار (PRV) یا شیر ایمنی فشار (PSV):
تفاوت این است که PSV ها دارای یک اهرم دستی برای فعال کردن شیر در مواقع اضطراری هستند. اکثر PRV ها فنری هستند. در فشارهای پایین تر، برخی از دیافراگم به جای فنر استفاده می کنند. قدیمی ترین طرح های PRV از یک وزنه برای آب بندی دریچه استفاده می کنند.
تنظیم فشار:
هنگامی که فشار سیستم به این مقدار افزایش می یابد، PRV باز می شود. دقت فشار تنظیم شده ممکن است از دستورالعمل های تعیین شده توسط انجمن مهندسین مکانیک آمریکا (ASME) پیروی کند.
شیر تسکین (RV):
دریچه ای که در سرویس مایع استفاده می شود، که با غلبه فشار فزاینده بر فشار فنر، متناسب با آن باز می شود.
سوپاپ ایمنی (SV):
مورد استفاده در سرویس گاز. اکثر SV ها کاملاً بالابر یا ضربه محکم هستند، به این ترتیب که کاملاً باز می شوند.
شیر ایمنی (SRV):
شیر تسکین دهنده ای که می تواند برای سرویس گاز یا مایع استفاده شود. با این حال، فشار تنظیم شده معمولاً فقط برای یک نوع سیال در یک زمان دقیق است.
شیر تسکین پایلوت (POSRV، PORV، POPRV):
دستگاهی که با فرمان از راه دور از یک شیر پایلوت که به سیستم بالادستی وصل است فشار را تخلیه می کند.
شیر ایمنی کم فشار (LPSV):
یک سیستم اتوماتیک که توسط فشار استاتیکی گاز کاهش می یابد. فشار تسکین دهنده کوچک و نزدیک به فشار اتمسفر است.
شیر ایمنی فشار خلاء (VPSV):
یک سیستم اتوماتیک که توسط فشار استاتیکی گاز کاهش می یابد. فشار تسکین دهنده کوچک، منفی و نزدیک به فشار اتمسفر است.
شیر ایمنی فشار کم و خلاء (LVPSV):
یک سیستم اتوماتیک که توسط فشار استاتیکی گاز کاهش می یابد. فشار تسکین دهنده کوچک، منفی یا مثبت و نزدیک به فشار اتمسفر است.
شیر خلاء فشار (PVRV):
ترکیبی از فشار خلاء و شیر تسکین در یک محفظه. در مخازن ذخیره مایعات برای جلوگیری از انفجار یا فشار بیش از حد استفاده می شود.
بازیگری سریع:
نقطه مقابل تعدیل، به دریچه ای اشاره دارد که باز می شود. در چند میلی ثانیه به طور کامل بالا می رود. معمولاً با یک دامن روی دیسک انجام می شود به طوری که مایعی که از صندلی عبور می کند به طور ناگهانی ناحیه بزرگ تری را تحت تأثیر قرار می دهد و نیروی بالابر بیشتری ایجاد می کند.
تعدیل:
متناسب با فشار بیش از حد باز می شود.

دانلود لوازم جانبی دوزینگ پمپ